Public Policy
The firings included 20 individuals in the FDA's Office of Neurological and Physical Medicine Devices.
The bill would allow AI to be classified as a "practitioner licensed by law" to administer FDA-approved drugs.
If finalized, the rule aims to improve the safety of patient data at healthcare institutions and addresses a national security topic by protecting data from bad actors who want to disrupt services, says Eric Avigdor, chief product officer of Votiro.
The EU guidelines highlight prohibitions on harmful manipulation, real-time biometric identification and social scoring.
AI, machine learning apps, educational tools, cybersecurity, workforce development, digital health transformation and new member benefits are among the highlights attendees can look forward to at HIMSS25, says HIMSS CEO Hall Wolf.
HIMSS25
Gunnar Trommer of BCG X and Erik Adams of BCG give highlights of their upcoming HIMSS25 panel on the capital needed to develop and commercialize AI-powered medical devices and on completing regulatory milestones like 510(k) clearance.
The U.S. Agency for International Development (USAID) administers civilian foreign aid and development assistance. It has released policies regarding digital health implementation and investment globally.
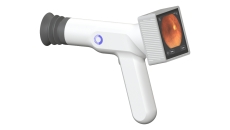
The Sentinel Camera allows point-of-care retinal imaging and eliminates the need for a visit to an eye specialist's office.
CMS raised the payment for homecare by only a half a percent, and that does not compare to inflation, says Luke Rutledge, president of Homecare Homebase, who adds: "That does not equate to the costs that continue to rise for our customers."
Ashkan Afkhami of BCG X sat down with MobiHealthNews during JPM to discuss the U.S. health system's strengths, challenges in cost control and the transformative potential of AI.