AI diagnosis
The AI, integrated into a hand-held point-of-care device, demonstrated 94% diagnostic accuracy.
LifeSemantics plans to commercialise its mobile app-based AI screening solution by yearend.
It uses a competitive learning approach against unlabelled data.
It is a simple and low-cost model that promotes the early detection of the condition.
The approval marks its entry into the $160-billion US medical device market.
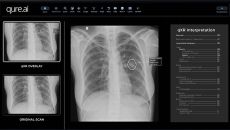
Qure.ai's qXR software will enable Mylab's upcoming x-ray device to quickly detect TB.
It seeks to raise the number of skin checks in Australia by a quarter.
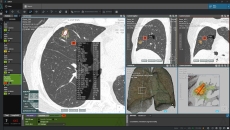
Also, Point Robotics from Taiwan has received a US FDA 510(k) clearance for its surgical robot system.
Also, a new online repository of health and wellness knowledge has been launched in India.
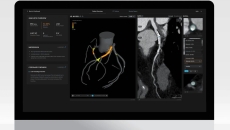
The clinical trial with Huntsville Heart Center is expected to be completed by October.